

(Remember left-handed alpha helices are not found in nature for similar reasons.) Summary of amino acids propensities for alpha helices (and beta structure as well) Those with branches at the beta carbon (Val, Ile) destabilize the alpha helix due to steric interactions of the bulky side chains with the helix backbone. The rest with no branches at the beta C can form helices.

The amino acids with side chains that can H-bond (Ser, Asp, and Asn) and aren't too long appear to act as competitors of main chain H bond donor and acceptors, and destabilize alpha helices. Gly is too conformationally flexible to be found with high frequency in alpha helices, while Pro is too rigid. Consider first those that aren't branched. Amino acids can be divided into two kinds, those with branches at the beta C and those with none.
Some amino acids are more commonly found in alpha helices than other. All the R-groups extend backward and away from the helix axis. There are not holes or pores in the helix. the core of the helix is packed tightly. the left-handed alpha helix, although allowed from inspections of a Ramachandran plot, is never observed, since the side chains are too close to the backbone. in proteins, the average number of amino acids in a helix is 11, which gives 3 turns. the alpha helix is more compact than the fully extended polypeptide chain with phi/psi angles of 180o. It can also be characterized by n (the number of amino acid units/turn = 3.6) and pitch (the helix rise/turn = 5.4 angstroms). The phi/psi angles for those amino acids in the alpha helix are - 57,-47, which emphasizes the regular repeating nature of the structure. These helices are formed when the carbonyl O of the i th amino acid H bonds to the amide H of the i th +4 aa (4 amino acids away). 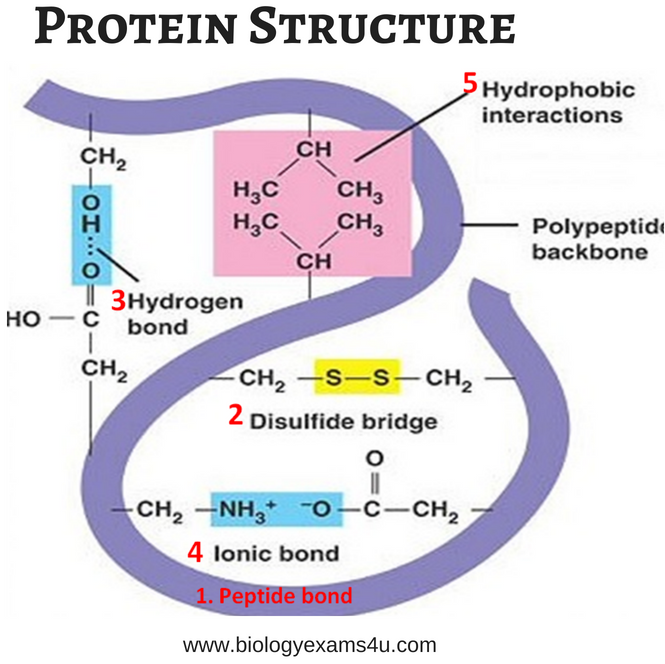
\)įigure: Right Handed Alpha helices - image made with VMD







 0 kommentar(er)
0 kommentar(er)
